Material Spotlight: Rogers BISCO® MS-1600 Series Medical Solid Silicone
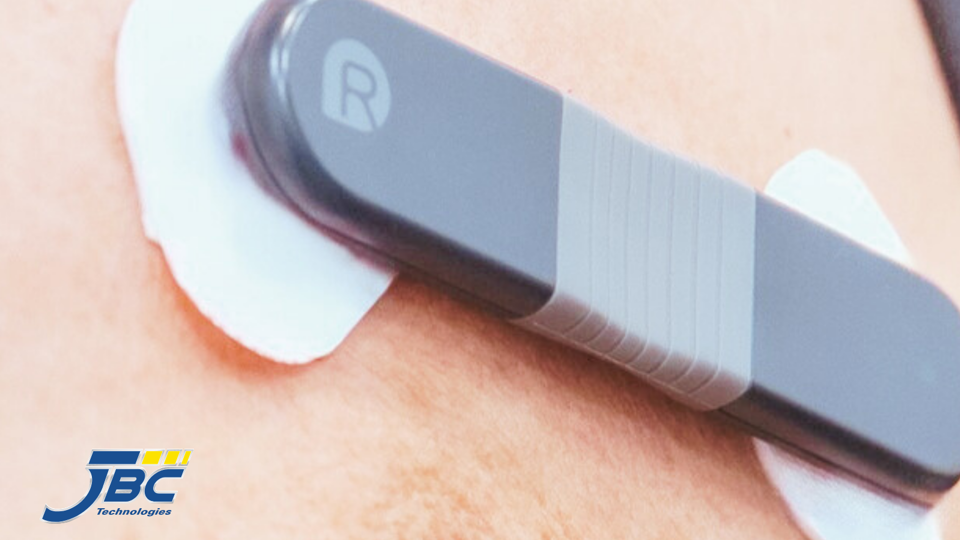
As innovations in wearable healthcare technologies continue to advance, medical wearables (and the parts and pieces that make them up) must be durable and able to endure exposure to moisture, chemicals, repetitive stress, and even temperature fluctuations. For more than 60 years, OEMs in the medical wearables space have turned to medical-grade solid silicones when they need a tough but flexible hypoallergenic material that can also achieve reliable, high-strength elastic bonds.
As part of JBC Technologies’ material spotlight series, today, we are highlighting Rogers Corporation BISCO® MS-1600, a premium medical-grade silicone intended for use in a variety of life science applications.
Key Characteristics of Medical Solid Silicones
Medical-grade solid silicones are an ideal solution for medical applications that will be reused, sanitized, or cycled many times. Silicone’s hypoallergenic and biocompatible nature makes it suitable for many life-science applications.
Some of the unique characteristics of medical-grade solid silicones include:
- Nonreactivity to surroundings, chemicals, temperature fluctuations, etc.
- Flexibility and durability in both dry and humid environments
- Strength and tear resistance while maintaining flexibility
- An ability to create tight seals in environments requiring tight tolerances
BISCO® Silicones MS-1600: A Biocompatible Solution for Medical Wearables
As a medical-grade solid silicone, BISCO® Silicones MS-1600 is a biocompatible product ideal for diagnostic consumables and test equipment, medical equipment in hospitals, and more. It provides excellent long-term sealing performance, biocompatibility, temperature and chemical resistance, and low levels of migratable silicones. Beyond the typical silicone advantages, the BISCO® MS-1600 line of products:
- Are highly resistant to UV, ozone, and most popular hospital sanitizers.
- Maintain high performance in a wide range of temperatures (-55 to 200 °C), including cycles of hot and cold.
- Are platinum-cured for safe and easy integration into applications.
- Are innately inert materials, compliant with the requirements of USP Class VI.
BISCO® MS-1600 is also produced with a low volatile, peroxide-free, platinum-cured raw material, which provides a non-toxic and chemically inert cured product.
Die-Cut Applications for Medical Solid Silicones
Die-cut applications for medical-grade solid silicones include medical device parts and products; pharmaceutical and bioprocessing parts and equipment; research, diagnostics, and PPE equipment; and more.
Medical-grade solid silicones can be converted to ultra-clean gaskets and seals for medical device parts, equipment displays, and wearable sensors or consumables.
Because silicone is customizable, many different types and grades are available. Selecting an experienced converting partner can help ensure you choose the best product for your application.
JBC: Much More than Precision Die-Cutting
Having trouble deciding what die-cut solution is right for your medical application? JBC Technologies can help. Our team provides expert guidance when it comes to material selection, part design, part presentation, assembly, and even manufacturing processes.
With two Class 8 Cleanrooms, state-of-the-art rotary and platen die-cutting equipment, and other vertically integrated processes, JBC Technologies is a leader in providing the highest-quality die-cutting, material converting, and contract manufacturing solutions for the medical industry.
Contact us today to learn how we can help you convert your design concepts into reality.